what needs to be done to convert a neutral nitrogen atom into an n3- species?
Chapter 7. Chemical Bonding and Molecular Geometry
7.3 Lewis Symbols and Structures
Learning Objectives
By the end of this section, yous volition be able to:
- Write Lewis symbols for neutral atoms and ions
- Draw Lewis structures depicting the bonding in unproblematic molecules
Thus far in this affiliate, nosotros have discussed the diverse types of bonds that form between atoms and/or ions. In all cases, these bonds involve the sharing or transfer of valence shell electrons betwixt atoms. In this department, we will explore the typical method for depicting valence beat out electrons and chemical bonds, namely Lewis symbols and Lewis structures.
Lewis Symbols
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded past one dot for each of its valence electrons:
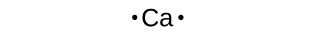
Figure 1 shows the Lewis symbols for the elements of the tertiary period of the periodic table.

Lewis symbols tin as well be used to illustrate the formation of cations from atoms, as shown hither for sodium and calcium:

Likewise, they can be used to show the formation of anions from atoms, as shown hither for chlorine and sulfur:

Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the germination of ionic compounds.

Lewis Structures
We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that depict the bonding in molecules and polyatomic ions. For example, when two chlorine atoms form a chlorine molecule, they share i pair of electrons:
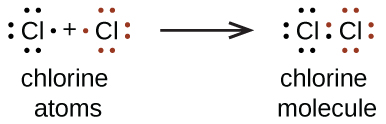
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written betwixt the atoms). A dash (or line) is sometimes used to betoken a shared pair of electrons:

A single shared pair of electrons is called a single bond. Each Cl cantlet interacts with eight valence electrons: the vi in the lonely pairs and the two in the single bail.
The Octet Rule
The other halogen molecules (F2, Brtwo, I2, and At2) course bonds similar those in the chlorine molecule: one single bond between atoms and iii lonely pairs of electrons per atom. This allows each halogen atom to take a noble gas electron configuration. The tendency of chief group atoms to course enough bonds to obtain eight valence electrons is known equally the octet rule.
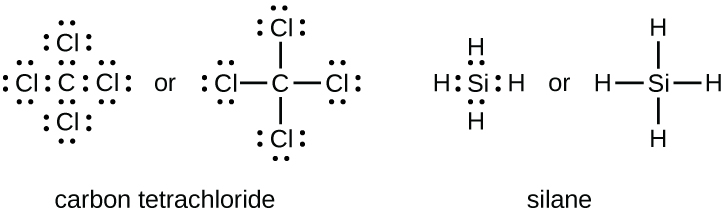
The number of bonds that an atom tin form can often be predicted from the number of electrons needed to reach an octet (8 valence electrons); this is especially true of the nonmetals of the second period of the periodic table (C, N, O, and F). For example, each cantlet of a group fourteen element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. These iv electrons can be gained past forming 4 covalent bonds, as illustrated here for carbon in CCl4 (carbon tetrachloride) and silicon in SiHiv (silane). Because hydrogen only needs two electrons to fill its valence trounce, information technology is an exception to the octet rule. The transition elements and inner transition elements besides do not follow the octet rule:
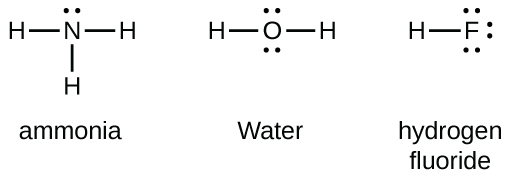
Group 15 elements such equally nitrogen take five valence electrons in the diminutive Lewis symbol: one alone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). Oxygen and other atoms in group sixteen obtain an octet by forming two covalent bonds:
Double and Triple Bonds
As previously mentioned, when a pair of atoms shares ane pair of electrons, we phone call this a single bond. However, a pair of atoms may need to share more than one pair of electrons in order to accomplish the requisite octet. A double bond forms when two pairs of electrons are shared between a pair of atoms, equally between the carbon and oxygen atoms in CHiiO (formaldehyde) and between the two carbon atoms in CtwoHfour (ethylene):
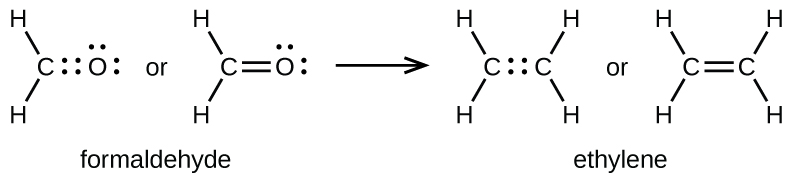
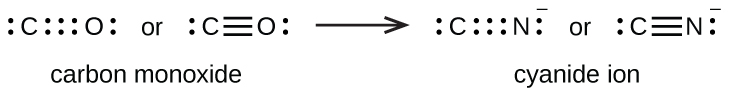
A triple bond forms when three electron pairs are shared by a pair of atoms, as in carbon monoxide (CO) and the cyanide ion (CN–):
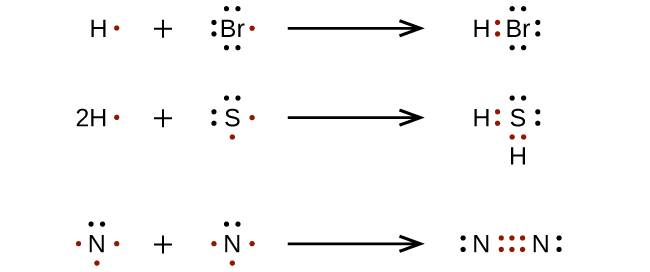
Writing Lewis Structures with the Octet Rule
For very elementary molecules and molecular ions, nosotros can write the Lewis structures by merely pairing up the unpaired electrons on the constituent atoms. See these examples:
For more complicated molecules and molecular ions, it is helpful to follow the step-past-step procedure outlined here:
- Determine the full number of valence (outer shell) electrons. For cations, subtract one electron for each positive charge. For anions, add one electron for each negative charge.
- Describe a skeleton construction of the molecule or ion, arranging the atoms effectually a fundamental atom. (Mostly, the least electronegative element should be placed in the heart.) Connect each atom to the central atom with a single bond (one electron pair).
- Distribute the remaining electrons as lonely pairs on the final atoms (except hydrogen), completing an octet effectually each atom.
- Place all remaining electrons on the central atom.
- Rearrange the electrons of the outer atoms to make multiple bonds with the central cantlet in club to obtain octets wherever possible.
Let us determine the Lewis structures of SiH4, CHO2−, NO+, and OF2 every bit examples in post-obit this procedure:
- Determine the full number of valence (outer crush) electrons in the molecule or ion.
- For a molecule, we add together the number of valence electrons on each cantlet in the molecule:
[latex]\begin{array}{r r l} \text{SiH}_4 & & \\[1em] & \text{Si: 4 valence electrons/atom} \times one \;\text{atom} & = four \\[1em] \rule[-0.5ex]{21em}{0.1ex}\hspace{-21em} + & \text{H: 1 valence electron/atom} \times 4 \;\text{atoms} & = 4 \\[1em] & & = 8 \;\text{valence electrons} \end{array}[/latex]
- For a negative ion, such as CHO2 −, we add the number of valence electrons on the atoms to the number of negative charges on the ion (one electron is gained for each single negative charge):
[latex]\begin{array}{r r l} {\text{CHO}_2}^{-} & & \\[1em] & \text{C: 4 valence electrons/atom} \times ane \;\text{atom} & = 4 \\[1em] & \text{H: ane valence electron/atom} \times 1 \;\text{atom} & = 1 \\[1em] & \text{O: half-dozen valence electrons/atom} \times 2 \;\text{atoms} & = 12 \\[1em] \dominion[-0.5ex]{21.5em}{0.1ex}\hspace{-21.5em} + & 1\;\text{additional electron} & = 1 \\[1em] & & = eighteen \;\text{valence electrons} \stop{array}[/latex]
- For a positive ion, such as NO+, we add the number of valence electrons on the atoms in the ion and then subtract the number of positive charges on the ion (one electron is lost for each unmarried positive charge) from the full number of valence electrons:
[latex]\brainstorm{assortment}{r r l} \text{NO}^{+} & & \\[1em] & \text{N: 5 valence electrons/cantlet} \times ane \;\text{cantlet} & = 5 \\[1em] & \text{O: 6 valence electrons/atom} \times 1 \;\text{atom} & = 6 \\[1em] \dominion[-0.5ex]{21em}{0.1ex}\hspace{-21em} + & -1 \;\text{electron (positive charge)} & = -1 \\[1em] & & = x \;\text{valence electrons} \end{array}[/latex]
- Since OFii is a neutral molecule, we only add the number of valence electrons:
[latex]\begin{array}{r r fifty} \text{OF}_{2} & & \\[1em] & \text{O: 6 valence electrons/atom} \times ane \;\text{atom} & = 6 \\[1em] \rule[-0.5ex]{21em}{0.1ex}\hspace{-21em} + & \text{F: seven valence electrons/atom} \times two \;\text{atoms} & = 14 \\[1em] & & = 20 \;\text{valence electrons} \cease{array}[/latex]
- For a molecule, we add together the number of valence electrons on each cantlet in the molecule:
- Draw a skeleton structure of the molecule or ion, arranging the atoms around a key atom and connecting each atom to the primal atom with a single (one electron pair) bond. (Note that nosotros denote ions with brackets around the structure, indicating the charge outside the brackets:)
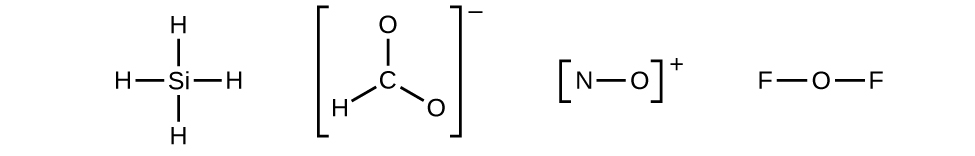 When several arrangements of atoms are possible, equally for CHOii −, we must use experimental evidence to choose the correct i. In full general, the less electronegative elements are more probable to exist central atoms. In CHOtwo −, the less electronegative carbon atom occupies the fundamental position with the oxygen and hydrogen atoms surrounding information technology. Other examples include P in POCl3, Due south in And soii, and Cl in ClOiv −. An exception is that hydrogen is almost never a primal cantlet. As the most electronegative chemical element, fluorine also cannot be a cardinal atom.
When several arrangements of atoms are possible, equally for CHOii −, we must use experimental evidence to choose the correct i. In full general, the less electronegative elements are more probable to exist central atoms. In CHOtwo −, the less electronegative carbon atom occupies the fundamental position with the oxygen and hydrogen atoms surrounding information technology. Other examples include P in POCl3, Due south in And soii, and Cl in ClOiv −. An exception is that hydrogen is almost never a primal cantlet. As the most electronegative chemical element, fluorine also cannot be a cardinal atom. - Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen) to complete their valence shells with an octet of electrons.
- In that location are no remaining electrons on SiH4, so it is unchanged:
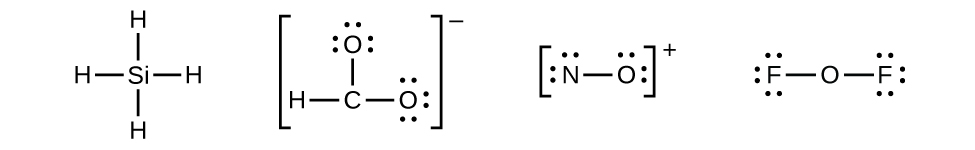
- In that location are no remaining electrons on SiH4, so it is unchanged:
- Identify all remaining electrons on the fundamental atom.
- For SiHiv, CHO2 −, and NO+, there are no remaining electrons; we already placed all of the electrons determined in Step i.
- For OF2, nosotros had 16 electrons remaining in Step 3, and we placed 12, leaving iv to be placed on the cardinal atom:
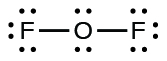
- Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
Example ane
Writing Lewis Structures
NASA's Cassini-Huygens mission detected a big cloud of toxic hydrogen cyanide (HCN) on Titan, one of Saturn's moons. Titan also contains ethane (HthreeCCH3), acetylene (HCCH), and ammonia (NHiii). What are the Lewis structures of these molecules?
Solution
- Summate the number of valence electrons.HCN: (ane × one) + (iv × 1) + (5 × 1) = 10H3CCH3: (1 × 3) + (2 × four) + (1 × 3) = 14HCCH: (1 × 1) + (2 × 4) + (1 × ane) = 10NH3: (five × 1) + (3 × i) = 8
- Depict a skeleton and connect the atoms with single bonds. Retrieve that H is never a central cantlet:
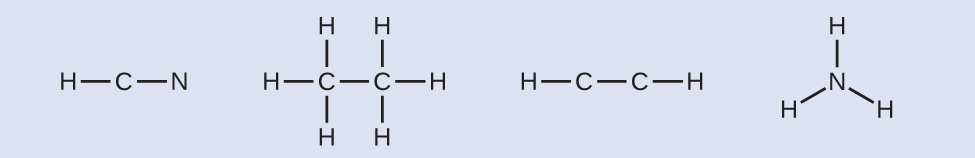
- Where needed, distribute electrons to the final atoms:
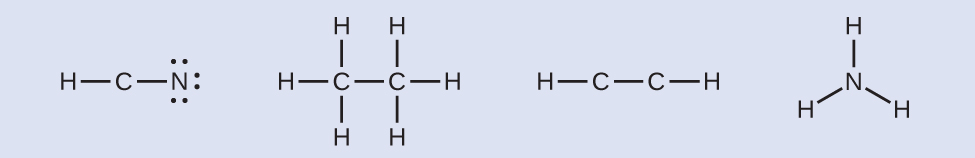 HCN: 6 electrons placed on NH3CCH3: no electrons remainHCCH: no final atoms capable of accepting electrons
HCN: 6 electrons placed on NH3CCH3: no electrons remainHCCH: no final atoms capable of accepting electrons NHthree: no final atoms capable of accepting electrons
- Where needed, place remaining electrons on the central cantlet:
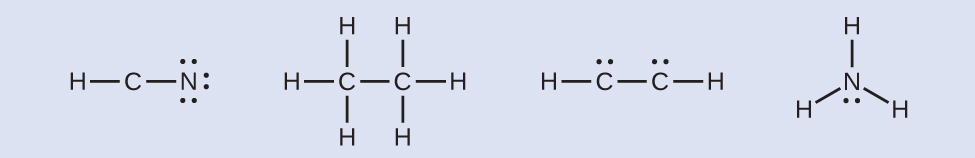 HCN: no electrons remainHthreeCCH3: no electrons remainHCCH: four electrons placed on carbon
HCN: no electrons remainHthreeCCH3: no electrons remainHCCH: four electrons placed on carbon NHiii: two electrons placed on nitrogen
- Where needed, rearrange electrons to form multiple bonds in order to obtain an octet on each atom:HCN: grade 2 more C–N bondsHthreeCCH3: all atoms have the right number of electronsHCCH: form a triple bond between the two carbon atomsNHthree: all atoms have the right number of electrons
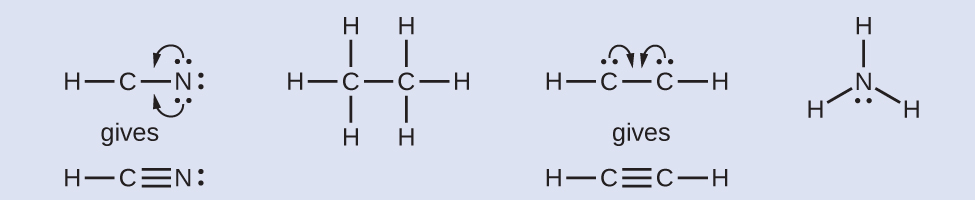
Check Your Learning
Both carbon monoxide, CO, and carbon dioxide, CO2, are products of the combustion of fossil fuels. Both of these gases also cause issues: CO is toxic and COii has been implicated in global climate alter. What are the Lewis structures of these ii molecules?
Respond:

Fullerene Chemistry
Carbon soot has been known to human since prehistoric times, only information technology was not until fairly recently that the molecular structure of the primary component of soot was discovered. In 1996, the Nobel Prize in Chemistry was awarded to Richard Smalley (Figure 3), Robert Curl, and Harold Kroto for their work in discovering a new form of carbon, the C60 buckminsterfullerene molecule (Figure ane in Chapter 7 Introduction). An unabridged form of compounds, including spheres and tubes of diverse shapes, were discovered based on Cthreescore. This type of molecule, called a fullerene, shows hope in a multifariousness of applications. Considering of their size and shape, fullerenes can encapsulate other molecules, so they take shown potential in various applications from hydrogen storage to targeted drug commitment systems. They also possess unique electronic and optical properties that have been put to good employ in solar powered devices and chemical sensors.

Exceptions to the Octet Dominion
Many covalent molecules have central atoms that do not have eight electrons in their Lewis structures. These molecules autumn into three categories:
- Odd-electron molecules have an odd number of valence electrons, and therefore have an unpaired electron.
- Electron-scarce molecules have a key atom that has fewer electrons than needed for a noble gas configuration.
- Hypervalent molecules have a central atom that has more electrons than needed for a element of group 0 configuration.
Odd-electron Molecules
We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an instance of an odd-electron molecule; it is produced in internal combustion engines when oxygen and nitrogen react at high temperatures.
To describe the Lewis structure for an odd-electron molecule similar NO, nosotros follow the same five steps we would for other molecules, simply with a few pocket-size changes:
- Decide the total number of valence (outer trounce) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = eleven. The odd number immediately tells us that we have a free radical, so we know that non every atom tin have eight electrons in its valence beat.
- Describe a skeleton construction of the molecule. We tin easily draw a skeleton with an North–O unmarried bond:N–O
- Distribute the remaining electrons as lone pairs on the final atoms. In this case, at that place is no central atom, so nosotros distribute the electrons around both atoms. We give eight electrons to the more electronegative atom in these situations; thus oxygen has the filled valence shell:
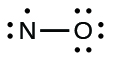
- Place all remaining electrons on the cardinal atom. Since there are no remaining electrons, this footstep does not use.
- Rearrange the electrons to brand multiple bonds with the central atom in order to obtain octets wherever possible. We know that an odd-electron molecule cannot take an octet for every cantlet, simply we desire to get each atom as close to an octet as possible. In this case, nitrogen has only five electrons around information technology. To motility closer to an octet for nitrogen, we have one of the lone pairs from oxygen and utilize it to form a NO double bail. (Nosotros cannot take another lone pair of electrons on oxygen and form a triple bond because nitrogen would then take 9 electrons:)
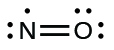
Electron-scarce Molecules
We will as well come across a few molecules that incorporate key atoms that do non have a filled valence beat. Generally, these are molecules with key atoms from groups two and 12, outer atoms that are hydrogen, or other atoms that practice not course multiple bonds. For instance, in the Lewis structures of beryllium dihydride, BeHtwo, and boron trifluoride, BF3, the beryllium and boron atoms each have only four and six electrons, respectively. It is possible to depict a construction with a double bond between a boron atom and a fluorine atom in BF3, satisfying the octet rule, simply experimental evidence indicates the bond lengths are closer to that expected for B–F single bonds. This suggests the best Lewis structure has three B–F unmarried bonds and an electron deficient boron. The reactivity of the compound is also consequent with an electron deficient boron. Still, the B–F bonds are slightly shorter than what is really expected for B–F single bonds, indicating that some double bond character is found in the bodily molecule.
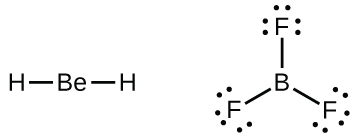
An atom like the boron atom in BFiii, which does not have 8 electrons, is very reactive. It readily combines with a molecule containing an atom with a lonely pair of electrons. For example, NH3 reacts with BF3 because the solitary pair on nitrogen can exist shared with the boron cantlet:
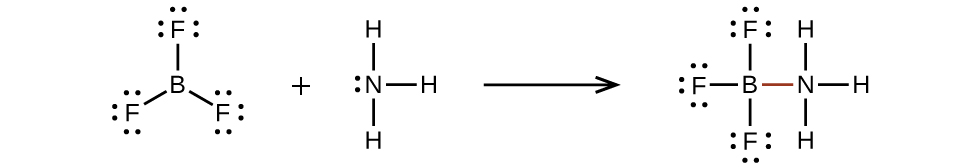
Hypervalent Molecules
Elements in the second period of the periodic tabular array (north = two) tin can adapt only viii electrons in their valence shell orbitals because they accept but four valence orbitals (one 2s and three 2p orbitals). Elements in the tertiary and higher periods (n ≥ three) accept more than four valence orbitals and can share more than four pairs of electrons with other atoms because they have empty d orbitals in the same crush. Molecules formed from these elements are sometimes called hypervalent molecules. Figure four shows the Lewis structures for two hypervalent molecules, PClv and SFhalf-dozen.
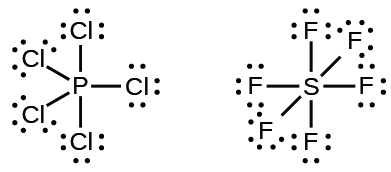
In some hypervalent molecules, such as IF5 and XeF4, some of the electrons in the outer shell of the central atom are lone pairs:

When we write the Lewis structures for these molecules, we find that we have electrons left over after filling the valence shells of the outer atoms with eight electrons. These additional electrons must exist assigned to the central atom.
Instance two
Writing Lewis Structures: Octet Dominion Violations
Xenon is a noble gas, but it forms a number of stable compounds. We examined XeFfour earlier. What are the Lewis structures of XeFii and XeF6?
Solution
We can describe the Lewis structure of any covalent molecule by post-obit the six steps discussed earlier. In this example, nosotros tin condense the last few steps, since not all of them apply.
- Calculate the number of valence electrons: XeF2: viii + (ii × 7) = 22XeF6: 8 + (6 × 7) = 50
- Draw a skeleton joining the atoms by single bonds. Xenon will exist the primal cantlet considering fluorine cannot exist a central cantlet:
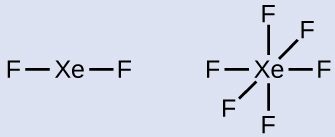
- Distribute the remaining electrons.XeFii: We place three lonely pairs of electrons around each F atom, accounting for 12 electrons and giving each F cantlet 8 electrons. Thus, vi electrons (three lone pairs) remain. These lone pairs must be placed on the Xe cantlet. This is acceptable because Xe atoms accept empty valence shell d orbitals and tin accommodate more than eight electrons. The Lewis structure of XeF2 shows 2 bonding pairs and three alone pairs of electrons around the Xe atom:
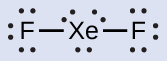 XeF6: We place three lone pairs of electrons around each F cantlet, bookkeeping for 36 electrons. 2 electrons remain, and this lone pair is placed on the Xe atom:
XeF6: We place three lone pairs of electrons around each F cantlet, bookkeeping for 36 electrons. 2 electrons remain, and this lone pair is placed on the Xe atom: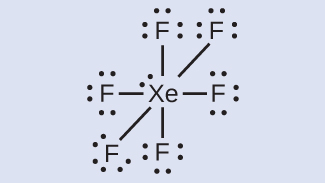
Cheque Your Learning
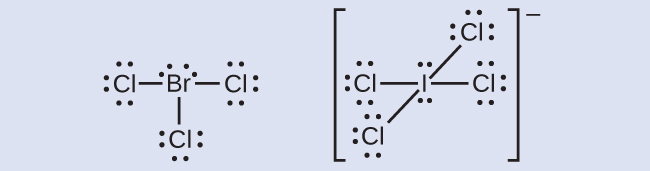
The halogens form a class of compounds chosen the interhalogens, in which halogen atoms covalently bond to each other. Write the Lewis structures for the interhalogens BrCl3 and ICl4 −.
Answer:
Primal Concepts and Summary
Valence electronic structures can exist visualized by cartoon Lewis symbols (for atoms and monatomic ions) and Lewis structures (for molecules and polyatomic ions). Alone pairs, unpaired electrons, and single, double, or triple bonds are used to indicate where the valence electrons are located around each atom in a Lewis structure. Most structures—especially those containing second row elements—obey the octet rule, in which every atom (except H) is surrounded past eight electrons. Exceptions to the octet dominion occur for odd-electron molecules (gratuitous radicals), electron-scarce molecules, and hypervalent molecules.
Chemistry End of Chapter Exercises
- Write the Lewis symbols for each of the following ions:
(a) Asthree–
(b) I–
(c) Be2+
(d) O2–
(eastward) Gathree+
(f) Li+
(1000) N3–
- Many monatomic ions are establish in seawater, including the ions formed from the following list of elements. Write the Lewis symbols for the monatomic ions formed from the following elements:
(a) Cl
(b) Na
(c) Mg
(d) Ca
(e) K
(f) Br
(g) Sr
(h) F
- Write the Lewis symbols of the ions in each of the following ionic compounds and the Lewis symbols of the atom from which they are formed:
(a) MgS
(b) Al2Oiii
(c) GaCl3
(d) K2O
(e) Li3N
(f) KF
- In the Lewis structures listed here, Grand and Ten represent various elements in the third period of the periodic table. Write the formula of each chemical compound using the chemical symbols of each element:
(a)
(b)
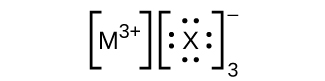
(c)
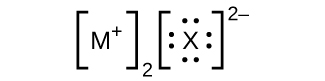
(d)
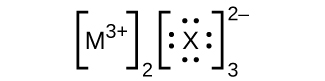
- Write the Lewis construction for the diatomic molecule Ptwo, an unstable form of phosphorus found in loftier-temperature phosphorus vapor.
- Write Lewis structures for the following:
(a) Hii
(b) HBr
(c) PClthree
(d) SFii
(due east) HiiCCH2
(f) HNNH
(g) HtwoCNH
(h) NO–
(i) N2
(j) CO
(one thousand) CN–
- Write Lewis structures for the following:
(a) O2
(b) HtwoCO
(c) AsFthree
(d) ClNO
(e) SiCl4
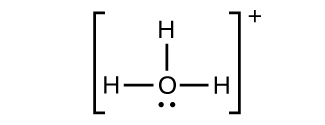
(f) H3O+
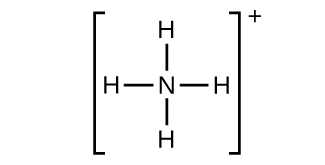
(thousand) NH4 +
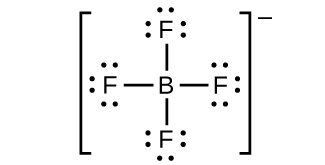
(h) BF4 −
(i) HCCH
(j) ClCN
(k) C2 2+
- Write Lewis structures for the following:
(a) ClF3
(b) PCl5
(c) BF3
(d) PFhalf dozen −
- Write Lewis structures for the following:
(a) SeFsix
(b) XeF4
(c) SeCl3 +
(d) CltwoBBClii (contains a B–B bond)
- Write Lewis structures for:
(a) PO4 3−
(b) ICl4 −
(c) Then3 2−
(d) HONO
- Correct the post-obit statement: "The bonds in solid PbCl2 are ionic; the bond in a HCl molecule is covalent. Thus, all of the valence electrons in PbClii are located on the Cl– ions, and all of the valence electrons in a HCl molecule are shared between the H and Cl atoms."
- Write Lewis structures for the post-obit molecules or ions:
(a) SbHthree
(b) XeFtwo
(c) Se8 (a cyclic molecule with a ring of 8 Se atoms)
- Methanol, H3COH, is used equally the fuel in some race cars. Ethanol, CtwoH5OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO2 and H2O when they burn. Write the chemic equations for these combustion reactions using Lewis structures instead of chemical formulas.
- Many planets in our solar organization comprise organic chemicals including methane (CHfour) and traces of ethylene (CtwoH4), ethane (C2H6), propyne (HiiiCCCH), and diacetylene (HCCCCH). Write the Lewis structures for each of these molecules.
- Carbon tetrachloride was formerly used in fire extinguishers for electric fires. It is no longer used for this purpose because of the formation of the toxic gas phosgene, CliiCO. Write the Lewis structures for carbon tetrachloride and phosgene.
- Identify the atoms that represent to each of the following electron configurations. Then, write the Lewis symbol for the common ion formed from each atom:
(a) 1south 22s ii2p 5
(b) isouth 22s 22p 6threedue south 2
(c) 1s 22southward iitwop half dozen3due south 23p half dozen4due south twothreed 10
(d) 1s twotwosouth two2p 6threes iithreep half dozen4s 23d ten4p 4
(due east) 1s 22s 22p 63southward two3p 64s ii3d 104p ane
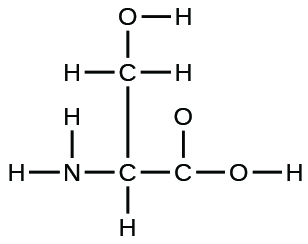
- The organization of atoms in several biologically of import molecules is given here. Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. Do not add any more atoms.
(a) the amino acrid serine:
(b) urea:
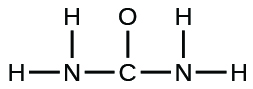
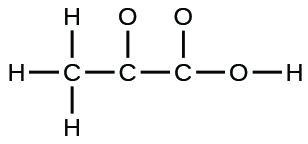
(c) pyruvic acrid:
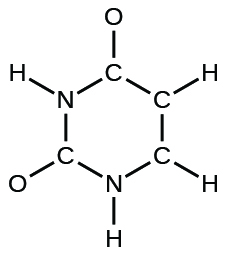
(d) uracil:
(due east) carbonic acid:
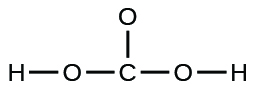
- A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound.
- A compound with a molar mass of well-nigh 42 thou/mol contains 85.7% carbon and 14.three% hydrogen past mass. Write the Lewis structure for a molecule of the compound.
- Two arrangements of atoms are possible for a compound with a molar mass of about 45 g/mol that contains 52.ii% C, 13.1% H, and 34.vii% O by mass. Write the Lewis structures for the two molecules.
- How are single, double, and triple bonds similar? How exercise they differ?
Glossary
- double bail
- covalent bail in which two pairs of electrons are shared between 2 atoms
- free radical
- molecule that contains an odd number of electrons
- hypervalent molecule
- molecule containing at least i master group element that has more than than eight electrons in its valence beat
- Lewis structure
- diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion
- Lewis symbol
- symbol for an element or monatomic ion that uses a dot to stand for each valence electron in the chemical element or ion
- lonely pair
- two (a pair of) valence electrons that are not used to course a covalent bond
- octet rule
- guideline that states master group atoms will grade structures in which eight valence electrons interact with each nucleus, counting bonding electrons as interacting with both atoms connected past the bond
- unmarried bond
- bond in which a unmarried pair of electrons is shared betwixt two atoms
- triple bond
- bond in which three pairs of electrons are shared between 2 atoms
Solutions
Answers to Chemistry End of Affiliate Exercises
one. (a) eight electrons:
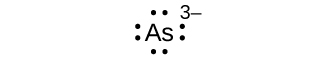 ;
;
(b) eight electrons:
 ;
;
(c) no electrons
Exist2+;
(d) eight electrons:
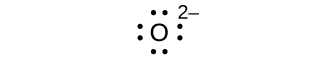 ;
;
(e) no electrons
Gathree+;
(f) no electrons
Li+;
(g) 8 electrons:
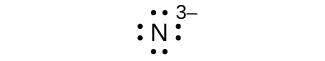
3. (a)
 ;
;
(b)
 ;
;
(c)
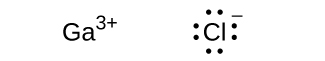 ;
;
(d)
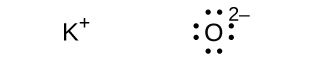 >;
>;
(e)
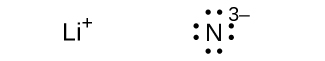 ;
;
(f)
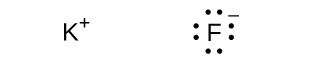
5.

7. (a)
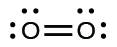
In this case, the Lewis structure is inadequate to describe the fact that experimental studies have shown two unpaired electrons in each oxygen molecule.
(b)
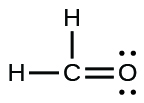 ;
;
(c)
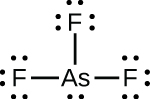 ;
;
(d)
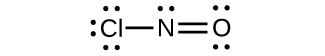 ;
;
(due east)
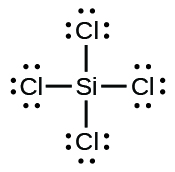 ;
;
(f)
 ;
;
(g)
 ;
;
(h)
 ;
;
(i)
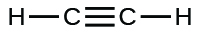 ;
;
(j)
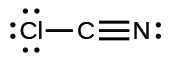 ;
;
(yard)
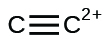
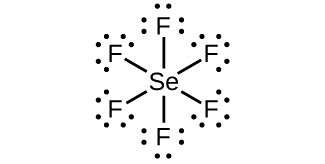
9. (a) SeF6:
 ;
;
(b) XeFfour:
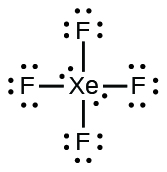 ;
;
(c) SeCliii +:
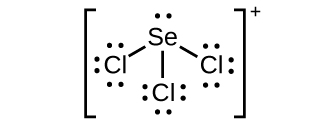 ;
;
(d) CltwoBBCl2:
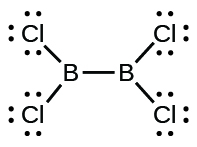
11. Ii valence electrons per Pb atom are transferred to Cl atoms; the resulting Leadii+ ion has a 6s 2 valence trounce configuration. Two of the valence electrons in the HCl molecule are shared, and the other six are located on the Cl cantlet as lone pairs of electrons.
13.

15.
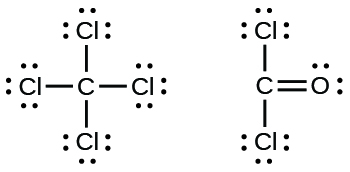
17. (a)
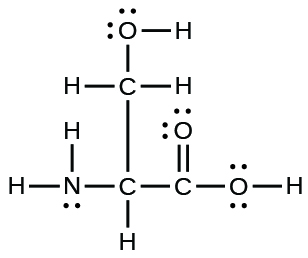 ;
;
(b)
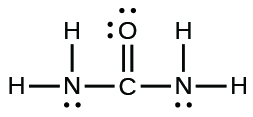 ;
;
(c)
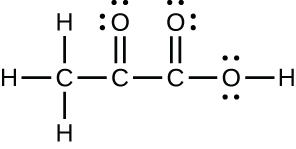 ;
;
(d)
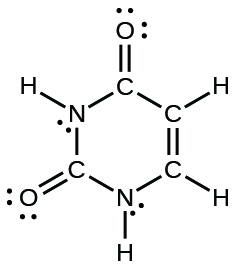 ;
;
(e)
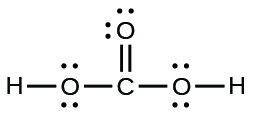
19.
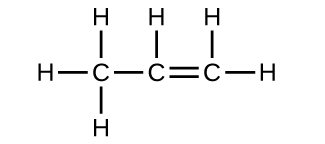
21. Each bond includes a sharing of electrons betwixt atoms. 2 electrons are shared in a unmarried bond; four electrons are shared in a double bond; and half dozen electrons are shared in a triple bail.
Source: https://opentextbc.ca/chemistry/chapter/7-3-lewis-symbols-and-structures/
0 Response to "what needs to be done to convert a neutral nitrogen atom into an n3- species?"
Post a Comment